Solid-state sensors
prepared by Katarzyna Wygladacz
In the conventional ion-selective electrodes (ISE), the ion-selective membrane is in electrical contact with the inner reference electrode through the inner reference solution. The convertion from ionic conductivity (in the membrane and the inner reference solution) to electron conductivity (in the inner reference electrode and external instrumentation) is provided by the reversible reaction of the inner reference electrode resulting in an ISE exhibiting a stable and reproducible standard potential.
The desire to miniaturise and mass fabricate sensors has led to the development of potentiometric solid-state sensors with new sensing systems, namely solid contact electrodes (SCEs) [1] such as solid crystal membranes and coated wire electrodes (CWEs) [2,3].
Coated-wire electrodes refer to a type of ISE in which an electroactive species is incorporated in a thin polymeric support film coated directly on a metallic conductor. This move to the total elimination of the internal filling solution provides new advantages. The substrate in the wire type electrodes is usually platinum wire, but silver, copper and graphite rods have also been used. CWEs are manufactured by dipping a metal wire into a solution of the membrane mixture [3]. The scheme of CWE is presented in the Fig. 1.
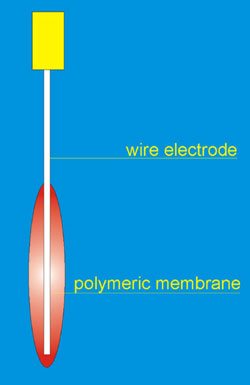
Fig. 1. The scheme of coated wire electrode
Sensors for Ca2+, NO3-, K+, Cl-, Li+ and ClO4- have been developed and sometimes these electrodes exhibited better selectivity than conventional type electrodes with an internal solution. Simplicity of design, lower costs, mechanical flexibility of miniaturisation and microfabrication widened the application for wire type electrodes, especially in the fields of medicine and biotechnology.
However, the configuration of CWE involves some crucial drawbacks. Commonly observed phenomena in such solid-state ion-sensors are a poor mechanical adhesion of the PVC-based sensitive layer to the transducer surface and insufficient electrochemical stability i.e. shift and drift of the EMF [4]. The standard potential of CWEs is often unstable varying for the electrode during its lifetime. These systems cannot provide very reproducible potentials due to the poorly defined charge transfer process at the interface between the ionically conducting membrane and the electrically conducting substrate. In the case of CWEs, drift characteristics were reported to be dependent on the kind of solids used [5,6] and on dissolved oxygen [6]. It has been found that usually an oxygen half-cell is set up on the metal surface so that the electrodes are susceptible to the oxygen content of the solution [7,8].
ISEs with direct contact of the membrane to a metal substrate constructed in a different manner have also been described. Some improvement has been made for this type of electrode with regard to the adhesion of the membrane by using a metal loaded epoxy as substrate [9], but these electrodes also did not possess a defined interface and were found to suffer from oxygen interface.
A more stable electrode potential can be obtained by contacting the ion-selective membrane to the solid substrate via an intermediate layer. Polymeric materials exhibiting mixed ionic and electronic conductivity, containing extended p-conjugated back-bones, such as poly(pyrrole) [10], poly(aniline) [11-13], poly(thiophene) [12] can be applied for this purpose. These materials may be prepared electrochemically by oxidation of their monomer.
Owing to the introduction of such electrically conducting polymers (CPs) t he ionic response of an ion-selective membrane (ISM) is converted to an electric signal.
To facilitate the charge transfer across the interface in solid contact ISE (SC-ISE), improve the potential stability and prevent oxygen interference the introduction SAM of a lipophilic redox-active compound was also proposed [14]. The structures of compounds used for this aim are presented in the Fig. 2.
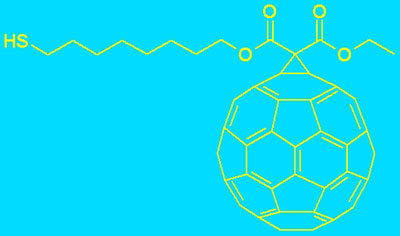
Fig. 2. The structures used compounds for SAM preparation
In such case a lipophilic and redox-active compound is attached by self-assembly to the inner gold electrode. The redox properties of the SAM guarantee a stable potential, while their lipophilicities prevent the formation of an aqueous layer between membrane and the metal electrode.
MINIATURISED SOLID-STATE SENSORS BASED ON SILICON TECHNOLOGY
The first miniaturized potentiometric solid-state sensors based on silicon technology were developed at University of Michigan several years ago [15,16]. Silicon structures employed for this purpose possessed both: the sensing and electrical contact sites on the front side of the chip (FSC). The scheme of such a sensor is presented in the Fig. 3.
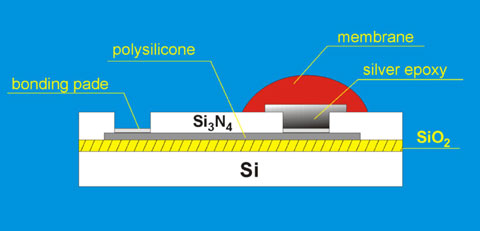
Fig. 3. The scheme of the front-side solid-state miniaturized sensor
Such sensors' design requires an additional encapsulation layer that covers the electrical contact pads. Insufficient quality of this encapsulation layer might cause a solid problem when sensors are used in an electrolyte solution. In order to eliminate this problem, potentiometric sensors with a back-side electrical contact (BSC) and front-side sensing site, fabricated with silicon technology, have been designed and applied for the fabrication of miniaturized BSC ion-sensors [17]. The structure of BSC sensor is presented in the Fig. 4.
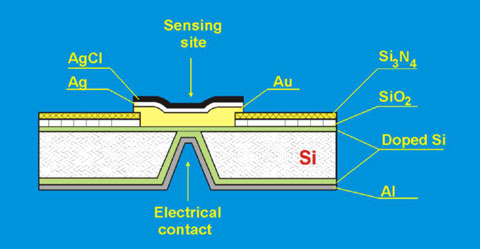
Fig. 4.The structure of BSC sensor
The back-side contact silicon-based chips were fabricated using IC technology, by photolithography processes. The structures possess the miniaturised Ag/AgCl sensing site situated on the front of the chip. Such Ag/AgCl electrode shows the changes in EMF as a function of chloride concentration according to the Nernst equation:
E = E0 - 59.16 lg aCl-
The potentiometric response (the calibration curves) of 10 randomly chosen sensors toward changes in chloride concentration is presented in the Fig. 5.
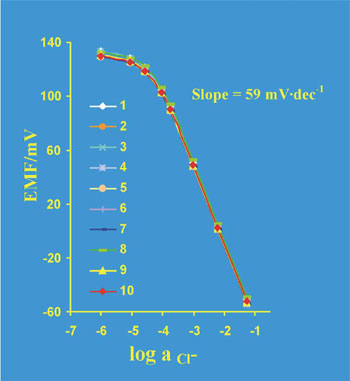
Fig. 5. The response of 10 randomly chosen BSC sensors toward changes in chloride concentration
Based on the potentiometric characteristics of BSC chip it can be concluded that Ag/AgCl sensing site can play role of the internal reference electrode. Such sensors are useful for miniaturised ion-selective sensor preparation.
However it was found that both kinds of sensors with front and back-side electrical contact suffer from instabilities of potential values. Several approaches (e.g. the incorporation of lipophilic silver-ligand complexes within polymeric films, the intermediate pHEMA layer introduction) have been suggested to improve the stability of such sensors by establishing a reversible electron transfer pair at the membrane/solid contact interface. The scheme of miniaturised solid-state chemically modified BSC sensor with intermediate pHEMA layer and polymeric membrane is presented in the Fig. 6.
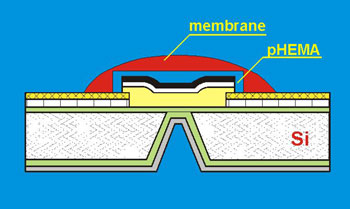
Fig. 6.The structure of BSC chemically modified sensor
Such configuration involves the charge transfer processes at both sides of the IS membrane well thermodynamically.
Ion-selective miniaturised BSC sensors with polymeric membranes are typically investigated in a galvanic cell:
Ag/AgCl/KCl(sat)/1M CH3COOLi//sample solution//liquid membrane/
/internal filing solution (pHEMA)/Ag/AgCl
It is common to divide the membrane potential (EM) into several separate potential contributions, namely the phase boundary potentials at both interfaces and the diffusion potential within the ion-selective membrane. The potential at the membrane/inner filling solution (pHEMA layer) interface can usually be assumed to be independent of the sample. The boundary potential (membrane/sample solution) depends on the ion-exchange processes between the solution and membrane phase. The diffusion potential within the membrane may become significant if considerable concentration gradients of ions with different mobilities arise in the membrane.
The selectivity of classical ISE and miniaturised sensors in the presence of the primary and interfering ions can be described by the selectivity coefficient according to the Nicolskii-Eisenman equation:
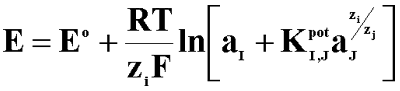
Where: E - the membrane potential; E0 - constant; aI, aJ - the activity of primary and interfering ions, respectively; ZI, ZJ - charge of primary and interfering ions, respectively; I - primary ions; J - interfering ion;
The miniaturised chemically modified back-side contact chips can be designed as anions as well as cations selective sensors. The calibration curves for NO2- - selective sensors with membranes based on linear polyurethane (Tecoflex) and containing the tetraphenyl porphyrin nitrite (CoTPPNO2) as a nitrite-selective ionophore are presented in the Fig. 7.
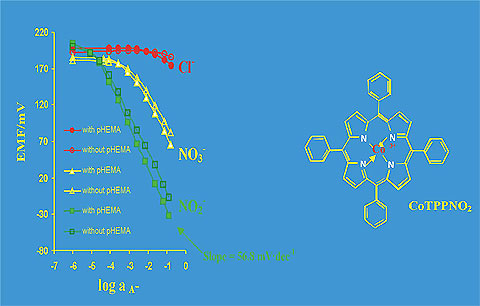
Fig. 7. The calibration curves for the BSC sensors based on Co(III)[TPP]NO2/TDMACl//PU/o-NPOE
The sodium selective chips based on back-side silicon sensors have also been described [18]. The potentiometric response of the BSC structures with polymeric membrane containing isodecyl acrylate/acrylinitryle and calix[4]arene as a sodium selective ionophore is presented in the Fig. 8.
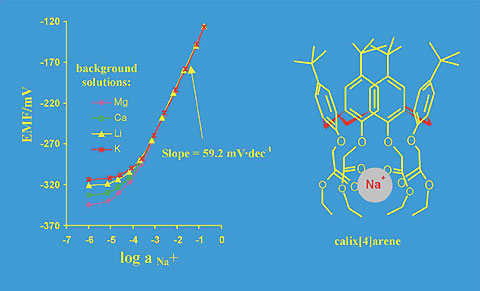
Fig. 8. The calibration curves of Na+ - selective miniaturised BSC sensors with intermediate pHEMA layer and membranes based on copolymer isodecyl acrylate/acrylonitryle and calix[4]arene as a sodium selective ionophore
Plasticised PVC is commonly used as the membrane material for classical ISEs and CWEs. However it can not be applied as a polymeric matrix for miniaturised silicon-based sensors due to the lack of its adhesion to Si3N 4 surface. Insufficient adhesion may cause detachment of ion-selective membrane based on linear polymer and shortening of sensor's lifetime. The application of the photocurable polymeric matrix is recommended in order to fulfil the requirements related to membrane adhesion to sensor's support and its mechanical properties.
REFERENCES
- B.P. Nikolskii, E.A. Materova, Ion Sel. Electrode Rev. 7, (1985), 3.
- J. Janata, Principles of Chemical Sensors, Plenum Press, New York, 1989.
- R.W. Cattrall, I.C. Hamilton, Ion Sel. Electrode Rev. 6, (1984), 125.
- H. van den Vlekkert, C. Francis, A. Grisel, N. de Rooji, Analyst 113, (1988), 1029.
- M. Maj-Zurawska, A. Hulanicki, Anal. Chim. Acta, 136, (1982), 395.
- M. Dror, E.A. Bergs, R.K. Rhodes, Sensors Actuators B 11, (1987), 23.
- A. Hulanicki, M. Trojanowicz, Anal. Chim. Acta, 87, (1976), 411.
- R.W. Cattrall, D.M. Drew, I.C. Hamilton, Anal. Chim. Acta 76, (1975), 269.
- S. Alegert, A. Florido, Analyst, 116, (1991), 473.
- A.F. Diaz, J.L. Cassillo, J.A. Logan, W.Y. Lee, J. Electroanal. Chem. 129, (1981), 115.
- E.M. Genies, A. Boyle, M. Lapkowski, C.P. Tsintavis, Synth. Met. 36, (1990), 139.
- J. Bobacka, T. Lindfors, M. McCarrick, A. Ivaska, A. Lewenstam, Anal. Chem. 67, (1995), 3819.
- T. Lindfors, A. Ivaska, Anal. Chim. Acta, 437, (2001), 171.
- M. Fibbioli, K. Badyopadhyay, S.G. Liu, L. Echegoyen, O. Enger, F. Diedriech, P. B ühlmann, E. Pretsch, Chem. Com. (2000), 339.
- H.D. Goldgberg, The Batch Fabrication of Integrated Chemical Sensors Arrays, Ph. D. Dissertation, University of Michigan, 1993.
- G.S. Cha, M.E. Meyerhoff, H.C. Cantor, A.R. Midgley, H.G. Goldberg, R.B. Brown, Anal. Chem. 63, (1991), 1666.
- K. Wygladacz, E. Malinowska, J. Jazwinski, Z. Brzozka, Sens. Actuators B, 83/1-3, (2002), 109.
- K. Wygladacz, E. Malinowska, J. Jazwinski, Z. Brzozka, SPIE, Vol 4616, (2001), 32.
|
Warsaw University of Technology Department of Analytical Chemistry Noakowskiego 3 00-664 Warsaw, Poland mail: brzozka@ch.pw.edu.pl phone: +48 22 234 5427 fax: +48 22 234 5631 www: csrg.ch.pw.edu.pl |


