Field effect transistors (FETs) as transducers in electrochemical sensors
prepared by Wojciech Wroblewski
INTRODUCTION
Chemical sensors are microdevices that connect the chemical and electrical domains (i.e. transduction of the chemical information into electric signal). The response of the sensors should be fast and selective for the analyte. Moreover, these devices should have a lifetime in the order of months. The construction of chemical sensors requires the integration of a sensing receptor and a transducing element into a defined chemical system. Field effect transistors (FETs) are very interesting because they can be made very small with current planar IC technology and have the advantage of a fast response time.
FROM MOSFET TO ISFET
The FETs are able to measure the conductance of a semiconductor as a function of an electrical field perpendicular to the gate oxide surface. In the most simple version, (i.e. a metal oxide semiconductor field effect transistor, n-channel MOSFET), a p-type silicon substrate (bulk) contains two n-type diffusion regions (source and drain). The structure is covered with a silicon dioxide insulating layer on top of which a metal gate electrode is deposited (figure 1a).
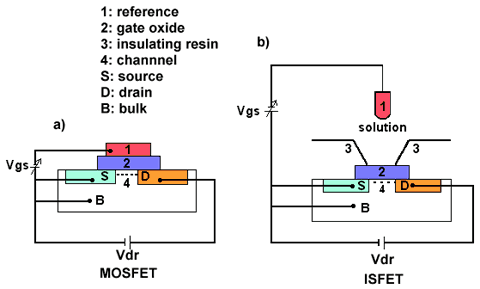
Figure 1. Schematic representation of a MOSFET a) and an ISFET structure b).
When a positive voltage (with respect to the silicon) is applied to the gate electrode, electrons (which are the minority carriers in the substrate) are attracted to the surface of the semiconductor. Consequently, a conducting channel is created between the source and the drain, near the silicon dioxide interface. The conductivity of this channel can be modulated by adjusting the strength of electrical field between the gate electrode and the silicon, perpendicular to the substrate surface. At the same time a voltage can be applied between the drain and the source (Vds), which results in a drain current (Id) between the n-regions.
In the case of the ISFET, the gate metal electrode of the MOSFET is replaced by an electrolyte solution which is contacted by reference electrode (then the SiO2 gate oxide is placed directly in an aqueous electrolyte solution, figure 1b) [1]. The metal part of reference electrode can be considered as the gate of the MOSFET.
In ISFET, electric current (Id) flows from the source to the drain via the channel. Like in MOSFET the channel resistance depends on the electric field perpendicular to the direction of the current. Also it depends on the potential difference over the gate oxide. Therefore, the source-drain current, Id, is influenced by the interface potential at the oxide/aqueous solution. Although the electric resistance of the channel provides a measure for the gate oxide potential, the direct measurement of this resistance gives no indication of the absolute value of this potential. However at a fixed source-drain potential (Vds), changes in the gate potential can be compensated by modulation of the Vgs. This adjustment should be carried out in such a way that the changes in Vgs applied to the reference electrode are exactly opposite to the changes in the gate oxide potential. This is automatically performed by ISFET amplifier with feedback which allow to obtain constant source-drain current. In this particular case, the gate-source potential, is determined by the surface potential at the insulator/electrolyte interface.
When SiO2 is used as the insulator, the chemical nature of the interface oxide is reflected in the measured source-drain current. The surface of the gate oxide contains OH-functionalities, which are in electrochemical equilibrium with ions in the sample solutions (H+ and OH-). The hydroxyl groups at the gate oxide surface can be protonated and deprotonated and thus, when the gate oxide contacts an aqueous solution, a change of pH will change the SiO2 surface potential. A site-dissociation model describes the signal transduction as a function of the state of ionization of the amphoteric surface SiOH groups [2,3]. Typical pH sensitivities measured with SiO2 ISFETs are 37-40 mV/ pH unit [3].
The selectivity and chemical sensitivity of the ISFET are completely controlled by the properties of the electrolyte/insulator interface. Other inorganic gate materials for pH sensors like Al2O3, Si3N4 and Ta2O5 have better than SiO2 properties in relation with pH response, hysteresis and drift. In practice, these layers are deposited on the top of the first layer of SiO2 by means of chemical vapour deposition (CVD).
ISFETs have been chosen as a transducing element because the SiO2 surface contains reactive SiOH groups which can be used for covalent attachment of organic molecules and polymers.
MEMFET AND SURFET
The ISFET can be modified with a sensing membrane, that contains an ionophore, which determines the response of the sensor [4]. If the gate oxide is covered with an ion-sensitive membrane, the device is known as a MEMFET [5]. In this case, the ion-sensing layer is penetrable for ions (unblocked); the membrane potential is generated throughout the membrane, which is detected by the FET structure. The first ISFET modified with a sensing membrane containing an ionophore, which enables the detection of the activity of an ion by its complexation, was reported by Moss [6]. A K+ - sensitive FET was obtained by solvent casting of a conventional plasticized PVC membrane, containing valinomycin on the gate oxide surface. Other approaches proposed Ca2+ sensitive MEMFET (with ion exchanger in polymeric membrane) [7] or deposition of AgBr membranes (Ag+ or Br- sensors) [8].
SURFET represents an ISFET with an ion-blocking layer, which covers the pH-sensitive sites of the gate insulator. At the surface of this layer a surface potential is established by selective association of ions. An example of a SURFET is the perylene gate ISFET with attached benzo-18-crown-6 ionophore molecules, that selectively complex potassium ions [9]. In contrast to the MEMFET, where the association coefficient of the ionophore with recognised ion in the membrane phase determines the selectivity, in SURFET the same process in the aqueous phase controls the selectivity.
It can be concluded from the literature reviews [10,11], that MEMFETs are readily fabricated by means of solvent casting of PVC membranes, with incorporated plasicizer and ionophore on the top of the ISFET gate oxide. Due to poor adhesion of the membrane to the gate oxide it can peel out easily and its electroactive components may leach out. The leaching out effect can be diminished by using extremely hydrophobic receptor or the ionophore can be covalently linked to the organic matrix at the ISFET gate oxide [12]. However, there is no thermodynamically well-defined membrane-ISFET interface and finally the pH sensitivity is not completely eliminated.
CHEMFET
ISFETs modified with plasticized PVC membranes lack a thermodynamically well-defined interface between the sensing membrane and the solid contact. Nevertheless, the PVC-modified ISFETs do not seem to suffer from the ill-defined inner contact and acceptable stabilities and drift values have been reported [7, 13, 14]. Up to now, no experimental efforts were made to improve this system because the properties of the devices are quite satisfactory. However, following studies showed, that changes of carbon dioxide concentrations in the sample solution influence strongly the measurements [15]. This was attributed to the diffusion of carbon dioxide through the membrane and the successive formation of carbonic acid at the membrane-gate oxide interface with traces of water present at the interface. Consequently, the concentration of protons, which determines potential at the membrane insulator interface, undergo large variations. This phenomenon explains why ISFETs modified with PVC membranes generally perform satisfactory (the PVC membranes usually contain reasonable amount of water and therefore H+ ions are present and control the membrane-insulator potential). Besides the CO2 interference, the need for high amount of water inside the membrane matrix was the key reason that urged to develop a thermodynamically well-defined interface.
Several approaches have been described in the literature for FET based sensors as possible solutions for these problems. In most cases, an intermediate Ag/AgCl layer is applied on the gate-insulator surface [16], which at least eliminates the CO2 interference. Various methods of deposition of a Ag/AgCl layer on a silicon substrate were reported with a conclusion, that different IC-compatible methods give satisfactory layers [17]. However, the Ag/AgCl-membrane interface becomes critical. The equilibrium state of this interface relies on the exchange of scarcely present Cl- ions in the membrane. Therefore, a better approach seems to be a deposition of an additional layer (e.g. sodium glass) between the polymer and the gate insulator or poly(vinyl alcohol) between the polymer and the Ag/AgCl layer on the top of the gate insulator [18]. In this way common ions can be provided by the intermediate layer.
Another approach, a novel architecture - chemically modified FET (CHEMFET), is designed to solve the problems (figure 2).
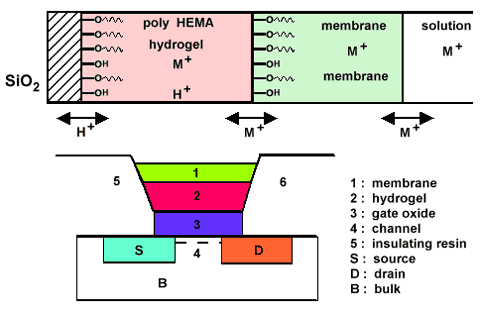
Figure 2. Schematic representation of a chemically-modified FET-CHEMFET.
Top: cross-section through the various layers with potential determining species.
The attachment of the membrane can be improved by mechanical [19] or chemical [20,21] anchoring to the surface of the gate oxide. For chemical attachment of polymer films the gate oxide surface is silylated with 3-(trimethoxysilyl)propyl methacrylate. The methacrylate modified surface can subsequently react with vinyl or methacryl monomers or prepolymers. The use of a UV-photopolymerizable monomers, hydroxyethyl methacrylate (HEMA), is advantageous from the point of view of the ultimately desired mass production of the CHEMFETs, which is essentially based on photolithography. The introduction of such a hydrogel layer [22,23], in which an aqueous buffered solution of salts can be absorbed, between the gate oxide and the sensing membrane eliminates the interference of CO2 on the CHEMFET response. Moreover, this stabilizes the potential developed in the sensing membrane. Plasticized PVC membranes, that contain an ionophore, are widely used as sensing membranes. Leakage of plasticizer to the contacting aqueous solution and weak adhesion of the membrane to the ISFET prompted the search for other polymer membranes like polyurethane, silicone rubber, polystyrene, polyamide and several polyacrylates [20,23,24].
The problem of the thermodynamically ill-defined membrane-gate interface was solved by an application of chemically attached poly(2-hydroxyethyl methacryalate) (polyHEMA) hydrogel between a hydrophobic membrane and the gate oxide layer. This novel architecture of FETs allows to design new chemical sensors based on polymeric membranes containing molecular receptors. CHEMFETs selective to K+ [20,25-27], Na+ [28-30], Ag+ [31], some transition metals cations (Pb2+, Cd2+) [32-34] and some anions (NO3-) [35-37] have been developed. However, the sensors exhibit limited lifetime which resultes from the leaching out of electroactive components, i.e. the ligand and the ionic sites. Electroactive components with an enhanced lipophilicity could be applied to increase durability of the sensor, but a more efficient method is based on covalent anchoring of these components to the membrane matrix. Application of membranes containing covalently bond ionophore and covalently bond ionic sites significantly improves the durability of CHEMFETs [26-29].
REFET
The use of a conventional reference electrode limites seriously the application of ISFETs with respect to the small size. Therefore the development of a miniature reference electrode made with the IC-compatible technology (reference field effect transistor - REFET) is of great interest for the wide-spread use of these sensors.
One of the approaches to solve this problem is the on-chip fabrication of an Ag/AgCl electrode with IC-compatible techniques, including a gel filled cavity and a porous silicon plug [38-42]. However, all the constructions have the disadvantage of a liquid-filled internal cavity with associated limited lifetime because of leakage of reference solution. A better approach to the problem of the reference electrode could be the application of two chemically unequally sensitive ISFETs operating in a differential mode with a common quasi-reference electrode (QRE) (e.g., a metal wire Pt), which can be easily integrated on the silicon chip [43-46]. This device have the additional advantage that external disturbances influencing both ISFETs (e.g. light and temperature) can be reduced. The accuracy of differential measurements depends on the difference in the ion sensitivity of both ISFETs, although total insensitivity of one ISFET (REFET) would be preferred. Such a reference FET should ideally case show insensitivity to all species present in the sample solution.
Originally, the oxide gate surface shows pH sensitivity, owing to the presence of hydroxyl groups, which can dissociate and can be protonated. It was reported that the total elimination of the pH-sensitive groups by chemical monolayer modification cannot be achieved [47]. However, the pH sensitivity can be suppressed by attaching to the gate surface an ion-blocking hydrophobic polymeric layer. In this modification the polymer is chemically bounded to the gate surface, which results in a long lifetime of the device. For ion-blocking layers, a stable attachment has been realized by plasma deposition [48-52]. However, potential variations with electrolyte compositions for such modified REFETs were observed [53]. Moreover, the deposition is limited to very thin polymeric layers because of diminishing electrical sensitivity (transconductance) with increasing insulator thickness [5].
In contrast to ion-blocking polymers, modification of REFETs with ion-unblocking (conductive) polymers would have the advantage of an equal transconductance of the REFET and ISFET [54,55]. Unfortunately, such ion-unblocking hydrophobic membranes result in a short lifetime of the sensor, if they are not chemically anchored to the surface. ISFETs have been modified in order to prepare REFETs with polymeric membranes which are covalently linked to the gate oxide surface [56,57].
Two types of REFET structures can be distinguished with respect to the penetration of ions into the polymeric layer, resulting in two different mechanisms of the REFET operation. In a non-ion-blocking REFET structure there is ion exchange between the solution and the polymer; consequently a thermodynamical equilibrium between ions in the solution and in the polymer is achieved and the membrane electrical potential is a membrane potential. In an ion-blocking REFET structure this ion exchange is negligible and in this case the electrical potential measured is a surface potential resulting from reversible ion-complexation reactions at the surface of the polymer.
CONCLUSION AND REFERENCES
Conclusion
The application of field effect transistors (FETs) as transducers in electrochemical sensors was firstly described in 1970 by Bergveld. These devices can transduce an amount of charge present on the surface of the gate insulator into a corresponding drain current. The fast expantion of these transducing elements was possible due to the introduction of IC-technology in their construction, which allowed mass fabrication.
This modern technology provide a possibility to design multi-ion sensors integrated with the reference cell (REFET). These sensors are very small, longliving and use only very small amounts of ion-sensing compounds.
References
- Bergveld P., IEEE Trans. Biomed. Eng., BME-17, 70 (1970).
- Bousse L., and Bergveld P., Sens. Actuators, 6, 65 (1984).
- van den Berg A., Bergveld P., Reinhoudt D.N., and Sudholter E.J.R., Sens. Actuators, 8, 129 (1985).
- Reinhoudt D.N., and Sudholter E.J.R., Adv. Mater., 2, 23 (1990).
- Bousse L., de Rooij N.F., and Bergveld P., IEEE Trans. Electron. Devices, ED- 30, 1263 (1983).
- Moss S.D., Janata J., and Johnson C.C., Anal. Chem., 47, 2238 (1975).
- McBride P.T., Janata J., Comte P.A., Moss S.D., and Johnson C.C., Anal. Chim. Acta, 101, 239 (1978).
- Buck R.P., and Hackleman D.E., Anal. Chem., 49, 2315 (1977).
- Matsuo T., Nakajima H., Osa T., and Anzai J., Proc. Transducers '85, Philadelphia, 1985, 250.
- Janata J., and Huber R.J., Ion-Selective Electrode Rev., 1, 31 (1979).
- Sibbald A., IEE Proc., 130, 233 (1983).
- Sudholter E.J.R., van der Wal P.D., Skowronska-Ptasinska M., van den Berg A., Bergveld P., and Reinhoudt D.N., Recl. Trav. Chim. Pays-Bas, 109, 222 (1990).
- Oesch U., Caras S., and Janata J., Anal. Chem., 53, 1983 (1981).
- Sibbald A., Whalley P.D., and Covington A.K., Anal. Chim. Acta, 159, 47 (1984).
- Fogt E.J., Untereker D.F., Norenberg M.S., and Meyerhoff M.E., Anal. Chem., 57, 1995 (1985).
- van den Vlekkert H.H., Decroux M., and de Rooij N.F., Proc, Transducers '87, Tokyo, 1987, 730.
- Dror M., Bergs E.A., and Rhodes R.K., Sens. Actuators, 11, 23 (1987).
- Talma A.G., Volders J.P.G.M., Ligtenberg H.C.G., Dost L., Bouwmeester H.J.M., and Boekema B., Sens. Actuators, 10, 35 (1987).
- Blackburn G.F., and Janata J., J. Electrochem. Soc., 129, 2580 (1982).
- van der Wal P., Skowronska-Ptasinska M., van den Berg A., Bergveld P., Sudholter E.J.R., and Reinhoudt D.N., Anal. Chim. Acta, 231, 41 (1990).
- Harrison D.J., Teclemariam A., and Cunningham L., Anal. Chem, 61, 246 (1989).
- Sudholter E.J.R., van der Wal P., Skowronska-Ptasinska M., van den Berg A., Bergveld P., and Reinhoudt D.N., Anal. Chim. Acta, 230, 59 (1990).
- Fiedler U., and Ruzicka J., Anal. Chim. Acta, 67, 179 (1973).
- Mascini M., and Palozzi F., Anal. Chim. Acta, 73, 375 (1974).
- Brzozka Z., Holterman H.A.J., Honig G.W.N., Verkerk U.H., van den Vlekkert H.H., Engbersen J.F.J., and Reinhoudt D.N., Sens. Actuators, 18-19, 38 (1994).
- Reinhoudt D.N., Engbersen J.F.J., Brzózka Z., van den Vlekkert H.H., Honig G.W.N., Holterman H.A.J., and Verkerk U.H., Anal. Chem., 66, 3618 (1994).
- van der Wal P.D., Sudholter E.J.R., and Reinhoudt D.N., Anal. Chim. Acta, 245, 159 (1991).
- Brunink J.A.J., Haak J.R.,Bomer J.G., Reinhoudt D.N., McKervey M.A., and Harris S.J., Anal. Chim. Acta, 254, 75 (1991).
- Brunink J.A.J., Bomer J.G., Engbersen J.F.J., Verboom W., and Reinhoudt D.N., Sens. Actuators, 15-16, 195 (1993).
- Brunink J.A.J., Lugtenberg R.J.W., Brzózka Z., Engbersen J.F.J., and Reinhoudt D.N., J. Electroanal. Chem., 378, 185 (1994).
- Brzozka Z., Cobben P.L.H.M., Reinhoudt D.N., Edema J.J.H., Buter J., and Kellogg R.M., Anal. Chim. Acta, 273, 139 (1993).
- Cobben P.L.H.M., Egberink R.J.M., Bomer J.G., Bergveld P., Verboom W., and Reinhoudt D.N., J. Am. Chem. Soc., 114, 10573 (1992).
- Cobben P.L.H.M., Egberink R.J.M., Bomer J.G., Schouwenaar R., Brzózka Z., Bos M., Bergveld P., and Reinhoudt D.N., Anal. Chim. Acta, 276, 347 (1993).
- Cobben P.L.H.M., Egberink R.J.M., Bomer J.G., Haak J.R., Bergveld P., and Reinhoudt D.N., Sens. Actuators, 6, 304 (1992).
- Dumschat C., Fromer R., Rautschek H., Muller H., and Timpe H.-J., Anal. Chim. Acta, 243, 179 (1991).
- Rocher V., Jaffrezic-Renault N., Perrot H., Chevalier Y., and Le Perchec P., Anal.Chim. Acta, 256, 251 (1992).
- Stauthamer W.P.R.V., Engbersen J.F.J., Verboom W., and Reinhoudt D.N., Sens. Actuators, 17, 197 (1994).
- Hochmair E.S., and Prohaska O., in W.H. Ko (Ed.), Implantable Sensors for Closed-Loop Prosthetic Systems, Futura, Mountkisco, NY, 1985, 652.
- Prohaska O., Goiser A., Jachomowits F., Kohl F., and Olcaytug F., Proc. of the 2nd International Meeting on Chemical Sensors, Bordeaux, 1986, 652.
- Smith R.L., and Scott D.C., Proc. of the Symposium on Biosensors, IEEE 84, CH-2068-5, 61 (1984).
- Smith R.L., and Scott D.C., IEEE Trans. Biomed. Eng., BME 33, 83 (1986).
- Margules G.S., Hunter C.M., and McGregor D.C., Med. Biol. Eng. Comput., 21, 1 (1983).
- Comte P.A., and Janata J., Anal. Chim. Acta, 101, 247 (1978).
- Hanazoto Y., and Shiono S., Chemical Sensors (Anal. Chem. Symp. Ser., Vol. 17), 513 (1983).
- Kuisl M., and Klein M., Messtechnik, 36, 48 (1983).
- Bergveld P., van den Berg A., van der Wal P.D., Skowronska-Ptasinska M., Suholter E.J.R., and Reinhoudt D.N., Sens. Actuators, 18, 307 (1989).
- van den Berg A., Bergveld P., Reinhoudt D.N., and Sudholter E.J.R., Sens. Actuators, 8, 129 (1985).
- Nakajima H., Esashi M., and Matsuo T., J. Electroanal. Chem. Soc., 129, 141 (1982).
- Fujihira M., Fukui M., and Osa T., J. Electroanal. Chem., 106, 413 (1980).
- Tahara S., Yoshii M., and Oka S., Chem. Lett., 1982, 307.
- Matsuo T., and Nakajima H., Sens. Actuators, 5, 293 (1984).
- Kimura J., Kuriyama T., and Kawana Y., Sens. Actuators, 9, 373 (1980).
- Matsuo T., and Esashi M., Extended Abstractsof the Electrochemical Society Spring Meeting, Seattle, WA, 1978, 202.
- Kimura J., Kuriyama T., and Kawana Y., Proc. Transducers '85, Philadelphia, 1985, 152.
- Cammann K., and Honold F., Proc. of the 2nd International Meeting on Chemical Sensors, Bordeaux, 1986, 554.
- Skowronska-Ptasinska M., van der Wal P., van den Berg A., Bergveld P., Sudholter E.J.R., and Reinhoudt D.N., Anal. Chim. Acta, 230, 67 (1990).
- van den Berg A., van der Wal P., Ptasin~ski D., Sudholter E.J.R., Reinhoudt D.N., and Bergveld P., Proc. of the 2nd International Meeting on Chemical Sensors, Bordeaux, 1986, 419.
|
Warsaw University of Technology Department of Analytical Chemistry Noakowskiego 3 00-664 Warsaw, Poland mail: brzozka@ch.pw.edu.pl phone: +48 22 234 5427 fax: +48 22 234 5631 www: csrg.ch.pw.edu.pl |


